By Fiona Mpofu
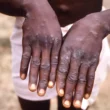
CLAIM: The Sinovac covid vaccine can cause poor blood circulation to one’s feet and hands. A Bulawayo woman claimed that she developed gangrene on her feet and hands after receiving her Covid-19 vaccine on 27 July 2021.
The health desk experts identified the condition as gangrene which can result from diabetes, peripheral artery disease and Raynauds disease (a health problem that causes decreased blood flow to the fingers.)
Currently, there is no evidence that the Sinovac vaccine causes any problems with blood circulation. Some of the side effects of the vaccine include pain at “the site of the injection,” headache, fatigue and muscle pain, and these resolve after 2 days.
The Strategic Advisory Group of Experts (SAGE) recommends continued monitoring post the administering of the covid19 vaccine.
“Among the observed potential severe side-effects of this vaccine are a severe allergic reaction known as Anaphylactic shock, a disorder causing inflammation of small blood vessels, facial paralysis, and a rare neurological disorder called acute disseminated encephalomy elitis,” they said.
“SAGE reported that they are “moderately” confident in the low risk of serious side effects after two doses in adults.”
They, however, reported less confidence in the risk of the older adults as there is less available data.
“SAGE currently recommends the use of Sinovac for individuals 18 years of age and older, including pregnant women, to prevent severe illness and hospitalization due to SARS-CoV-2 infection.”
The vaccine can be safely administered to individuals between the ages of 18 and 59, there is however little information on its safe administration to 60 years and older individuals.
“Due to the small number of individuals in that age group in the clinical trials, there is currently not enough data to suggest whether the vaccine is safe or not. The current recommendation is for the vaccine to be administered in two doses, two to four weeks apart.”