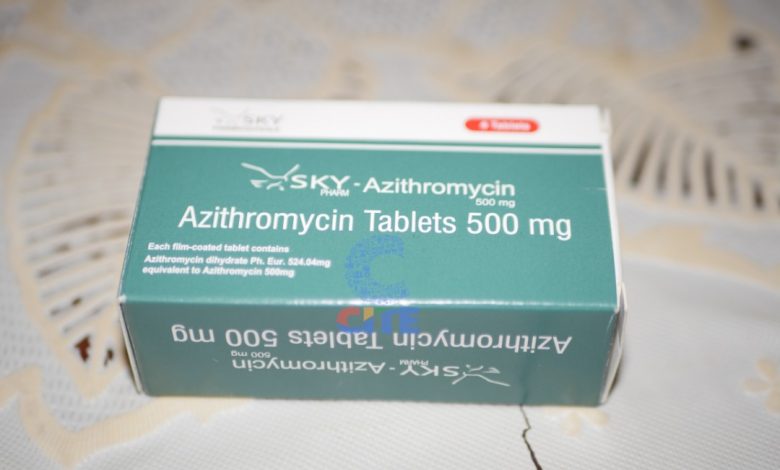
Investigations done by CITE revealed that the medication was issued before the expiry date had lapsed. Some of the residents received the pills on June 29, a day before the expiry date of 30 June, 2020.
City council health committee chairperson Cllr Lilian Mlilo told CITE in an interview that the tablets were still effective and could function properly for at least three more months after the expiry date.
“We have spoken to a doctor about the issue. There is nothing wrong with the medication that the people were given. The pills may have expired but it does not mean that they would automatically cease functioning. They remain safe and functional for the next three months. The residents should not be afraid to take the medication,” Cllr Mlilo said.
Health professional and Secretary of the Zimbabwe Association of Doctors for Human Rights (ZADHR) Norman Matara said in the event medication lapses its expiry date, it has to be taken to be sent to Medicine Control Authority of Zimbabwe for quality assessment.
“Some medication can indeed remain effective even after the expiry date lapses. However, under such circumstances, the medication needs to be taken to the Medicine Control Authority of Zimbabwe where the quality assessment of the drug can be conducted. This authority then certifies the effectiveness of the medication and issues an extension of the expiry date,” Dr Matara explained.
Responding to emailed questions from CITE, the Medical Control Authority of Zimbabwe (MCAZ) noted that unless stability data supporting use beyond 30th of June 2020 is submitted, the Authority does not recommend use beyond this expiry date because it is not guaranteed that the product will retain the same safety, quality and efficacy profile beyond 30th of June 2020. Having said that, it is possible to extend the shelf life beyond the expiry date.
MCAZ further explained that shelf-life extension programs exist within the pharmaceutical sector only based on rigorous RISK-BENEFIT analysis.
“During the manufacture of the medicine, various studies are conducted to check whether the Finished Pharmaceutical Product (FPP), containing the API and the various ingredients (excipients), will remain stable over a sustained period of time,” MCAZ noted.
“The purpose of these stability studies is to determine whether the medicine’s potency and characteristics will be maintained, whilst the medicines are being distributed, stored and used by the end-user, that is, it aims to ensure that throughout the medicine’s “life-cycle”, it will remain safe and retain its quality characteristics.”
Background: Luveve residents have for the past two months been battling with an outbreak of diarrhoea which has to date claimed 13 lives.
The local authority, in a bid to curb further loss of life in the community, offered free medical attention to all residents affected by the diarrhoea.
The residents were offered Azithromycin Tablets medication which is meant to kill the bacteria.