VERDICT :Chinese authorities gave conditional approval for general public use of the Sinopharm vaccine, interim data relased by the Sinopharm drug company showed that the vaccine had a 79% efficacy rate in phase three trials, at least 17 countries have purchased China-produced COVID-19 vaccines
The deputy commissioner of China’s National Medical Products Administration, Chen Shifei, announced the decision at a news conference in Beijing on 31 December 2020.
“After a series of strict reviews, verification, test and data analysis in accordance with the law and procedures, it is concluded that the known and potential benefits of Sinopharm’s new inactivated coronavirus vaccine are bigger than the known and potential risks, and it fully meets the pre-set requirements of conditional marketing standards,” he said
Sinopharm submitted study data and safety, efficacy, and quality information to the WHO in December 2020. The assessment is ongoing and a decision could be made as early as March.
Zimbabwe received 200,000 doses of China’s Sinopharm vaccine, donated by Beijing to help halt the spread of COVID-19. China’s ambassador to Zimbabwe Guo Shaochun dismissed any doubts about the Sinopharm vaccine.
“I am not a scientist, so I do not make unprofessional comments. But I would like to say: the safety, the Chinese vaccine is already proven,”
Asked if the Sinopharm Vaccine may have side effects @China_Amb_Zim says This question requires and expert but we should know that every drug has side effects. Most people may have pain or fever or some other mild reactions
Zimbabwe had vaccinated a total number of people so far to 18 843.
Bellow are the graphs of countries where the Sonopharm vacine is being used
What our experts say
As of February 19, 2021, the World Health Organization (WHO) has approved the Pfizer/BioNTech COVID-19 vaccine (December 31, 2020) and two versions of the AstraZeneca/Oxford COVID-19 vaccine (February 15, 2021) under its Emergency Use Listing (EUL).
The Sinopharm inactivated virus COVID-19 vaccine is being developed and produced in conjunction with the China National Biotec Group and the Beijing Institute of Biological Products. The vaccine is in phase three trials in Argentina, Jordan, Egypt, Bahrain, and United Arab Emirates. Sinopharm is pursuing emergency approval and, according to WHO records dated January 20, 2021, Sinopharm submitted study data and safety, efficacy, and quality information to the WHO in December 2020. The assessment is ongoing and a decision could be made as early as March.
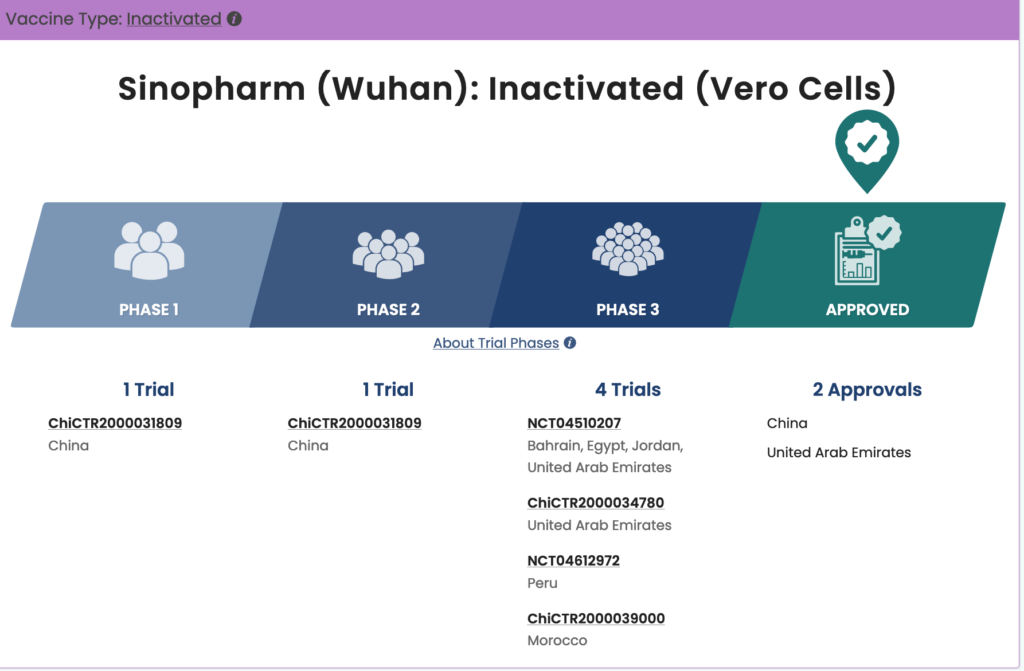
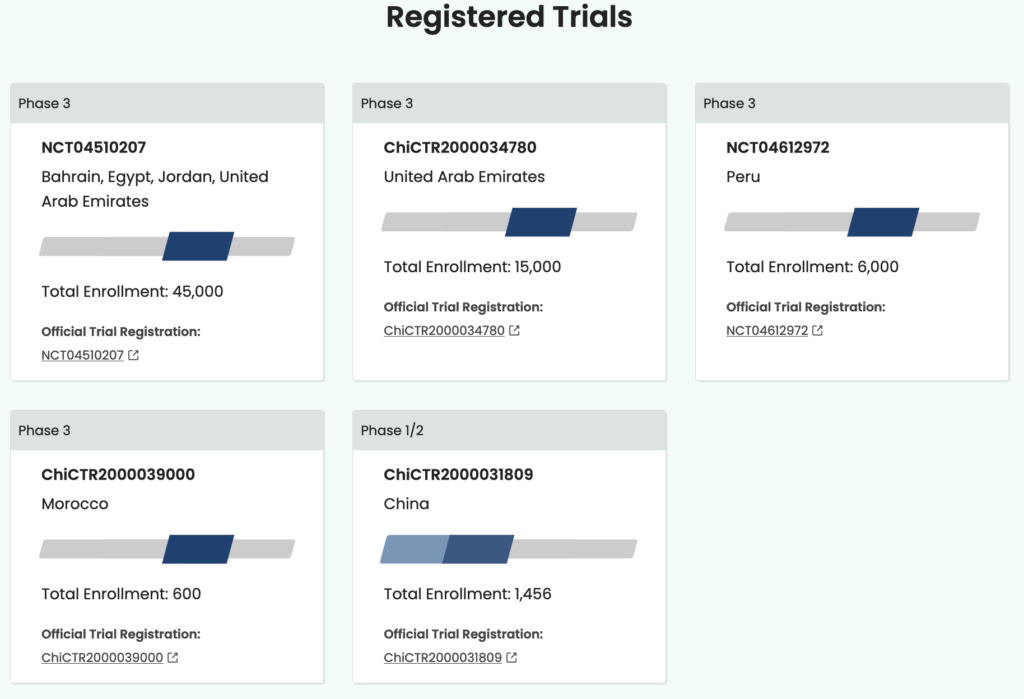
Another Sinopharm COVID-19 vaccine is being tested in conjunction with the China National Biotec Group and the Wuhan Institute of Biological Products. This vaccine is currently in phase three trials in Jordan, Egypt, Bahrain, Morocco, Peru, and United Arab Emirates. According to WHO documentation dated January 20, 2021, there has not been a pre-submission meeting or further pursuit of emergency approval for this vaccine.
Context and background
The World Health Organization (WHO) may approve medications, vaccines, or other health products for Emergency Use Listing (EUL) during public health emergencies, with the goal of making these new therapies available as soon as possible. The approval process includes a very strict review of safety, efficacy and quality as well as the potential risks and benefits of the product’s potential use.
During the COVID-19 pandemic, WHO emergency approval is required before the vaccine can be considered for COVAX Facility vaccine supply. The WHO emergency approval also allows “countries to expedite their own regulatory approval to import and administer COVID-19 vaccines.”
Even once a vaccine has received emergency approval from the WHO, the WHO will continue to monitor and assess data from vaccine trials and vaccine use in the general population. This monitoring and assessment process is to ensure that the vaccines meet required quality, safety, and efficacy standards.